Saccharomyces boulardii is unique in this database of the world's best probiotic strains, in that it is a non-pathogenic, transient (non-colonising) yeast, originally extracted from the lychee fruit, rather than a bacteria. It is important to note, however, that it is genetically and functionally different from the well-known pathogenic Candida family of yeasts. It was originally classified as a separate species but, after much laboratory testing, it was shown to have certain genomic and structural similarities to Saccharomyces cerevisiae and so is now classified as part of this species. That being said, S. boulardii is a unique microorganism and has vastly different strain-specific properties to other yeasts in the same S. cerevisiae species, in the same way that different strains of bacteria may have completely different actions and properties to other strains within the same species. For more information on the classification of Saccharomyces boulardii, please read our in-depth article.
S. boulardii is the only yeast that has been formally recognised as a probiotic, and it has multiple mechanisms by which it confers benefits to its host. It is recognised as having a broad anti-pathogenic action, due to its sticky outer surface which attaches to pathogenic bacteria such as Escherichia coli and Salmonella, and removes them from the digestive system. As a yeast itself, a much larger micro-organism than a bacterium, S. boulardii is able to effectively compete with and displace harmful yeast strains such as Candida. As part of its survival technique, it also produces anti-fungal substances such as capric, caprylic, and caproic acids, which discourage pathogenic yeasts in its environment.
In addition to this anti-pathogenic action, S. boulardii offers other benefits to its host as it passes through the digestive system. It increases the brush border enzymes in the intestinal wall, e.g. alkaline phosphatases and a-glucosidases, so may help to optimise nutrient absorption. It also secrets an enzyme (leucine aminopeptidase) that helps to prevent allergies to common dietary proteins developing after gut damage resulting from gastroenteritis. Finally, it promotes the secretion of the immunoglobulin secretory IgA (sIgA), a vital part of our immune defences against incoming pathogens and toxins, which helps to downregulate the action of pro-inflammatory cytokines pathways generated in response to pathogenic bacteria, toxins and antigens (Qamar et al, 2001).
Saccharomyces boulardii is one of the most researched probiotics in the world and takes its name from the French scientist, Henri Boulard. Boulard discovered the probiotic yeast in 1923 in South East Asia, where he noticed people using the skin of lychee fruit for its beneficial health properties. S. boulardii is often referred to as S. boulardii lyo, where the 'lyo' is short for 'lyophilisation' - a form of freeze-drying which S. boulardii normally undergoes in its supplement form. This process allows the supplement to be shelf-stable so that refrigeration is not necessary.
Saccharomyces boulardii is a food supplement commonly used in Europe with research demonstrating its safety and survival to reach the gut alive. There have been numerous meta-analyses of this probiotic yeast to determine efficacy and safety (Mc Farland, L., 2010; Kelesisis, T. & Pothoulakis, C., 2012). It has been deemed safe and well tolerated in many clinical trials, however it should be noted that those who are severely immune compromised or those requiring central venous catheters are advised to only take this strain under medical supervision, as in rare cases fungaemia has been reported.
S. boulardii has been isolated in stools when taken orally, as assessed by Klein, S. M. et al., (1993) who identified recovery in the stool in a dose dependant manner, and Blehaut, H. et al., (1989). This indicates that S. boulardii survives to reach the gut alive.

Saccharomyces boulardii is probably best known for its potential to relieve diarrhoea symptoms; it has been found to reduce antibiotic-associated diarrhoea (AAD), traveller’s diarrhoea, IBS-D, and acute or chronic diarrhoea in children and in adults. Its anti-diarrhoeal properties are so well-recognised and researched that it is used routinely as an anti-diarrhoeal medicine in many hospitals around the world. Saccharomyces boulardii is actually a registered medicine in over 100 countries in the world, under the brand name Florastor.
McFarland, L. V. (2010) conducted a systematic review and meta-analysis of Saccharomyces boulardii in adult patients, looking at the efficacy and safety of Saccharomyces boulardii (S. boulardii) for various disease indications in adults. This was based on the peer-reviewed, randomised clinical trials and pre-clinical studies from the published medical literature between 1976 and 2009. This review of 31 randomised, placebo-controlled treatment arms in 27 trials (encompassing 5029 study patients), found S. boulardii to be significantly effective and safe in 84% of those treatment arms. A meta-analysis found a significant therapeutic efficacy for S. boulardii in the prevention of antibiotic-associated diarrhoea. Saccharomyces boulardii can therefore be strongly recommended for prevention of AAD, as well as for traveller’s diarrhoea and the prevention of nutrition-related diarrhoea; also for the alleviation of Helicobacter pylori infection and related symptoms.
To further demonstrate the efficacy of S. boulardii for the prevention of antibiotic-associated diarrhoea symptoms, a total of 269 children with otitis media (an inflammatory disease of the middle ear), and/or respiratory tract infections were enrolled to take part in a randomised, double-blind, placebo-controlled trial. For the purposes of the trial, one group of children was given antibiotic treatment, along with a supplement containing 5 billion CFU of S. boulardii, whilst another group was given the antibiotic treatment along with a placebo supplement. The results indicated that the group taking the probiotic had a lower incidence of diarrhoea than the group taking the placebo (Kotowska et al., 2005).
A further related double-blind, placebo-controlled trial was conducted with the participation of 130 children aged 3 months to 3 years, all of whom were suffering with diarrhoea symptoms. The group of children was divided into two sub groups, a treatment group being given a supplement containing 400 million CFU of Saccharomyces boulardii dissolved in 5mg of liquid and the second control group given a liquid placebo supplement. The results showed that S. boulardii made a significant impact on the rate of recovery from diarrhoea. The method of action was identified as its inhibitory effect on the growth of pathogenic strains (Cetina-Sauri, 1994).
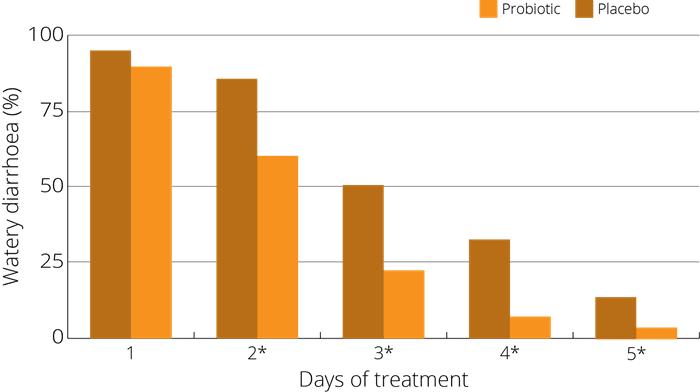
A study (Wan et al 2017) on children was run to test the efficacy and safety of S. boulardii in preventing AAD in 1-3 year old children - 408 cases divided into groups. One being given S. boulardii alongside antibiotics, and the other not. Whilst on antibiotics, the incidence of AAD in prevention group was 10.3% (22 cases) which was significantly lower than that of control group 29.2% (57 cases). Additionally, post antibiotics a difference was also seen, with new cases of diarrhea in prevention group being 2.4% (5/213) which was significantly lower than that in control group 16.4% (32/195).
In another trial, 60 children with watery diarrhoea and the rotavirus were randomised into a control and test group. The test group was given S. boulardii for 5 days. The result was that the average duration of diarrhoea was shorter in the intervention group (60 vs 89) and there was also a significantly shorter duration of hospital stay (74 vs 91) (Das S, et al., 2016).
A 2021 meta-analysis of 84 studies involving 13,443 children found that certain single-strain probiotics effectively treated acute diarrhea in children with various certainty evidence. Researchers concluded that among all probiotics, Saccharomyces boulardii may be the most effective in reducing both duration of diarrhea (compared with placebo) and risk of diarrhea lasting ≥2 days (compared with placebo or no treatment), with moderate evidence. (Zengbin L, et al., 2021)
Further relevant studies: Abbas et al (2014), Akil et al (2006), Bruggengate (2015), Burande (2013), Can et al (2006), Canani et al (2007), Cindoruk M., (2007), Corrêa et al (2011), Cremonini et al. (2002), Dinleyici et al., (2015), Doron et al., (2008), Duman et al., (2005), D'Souza A. et al., (2002), Eren et al (2010), Fidan I. et al., (2009), Gaón D.l. et al., (2003), Gotteland M. et al., (2005), Grandy G. et al., (2010), Hafeez et al (2002), Hochter W. et al., (1990), Htwe K. et al. (2008), Hurduc et al. (2009), Kabir et al (2009), Kirchhelle A. et al., (1996), Kurugol Z. & Koturoglu, G. (2005), Le Luyer B. et al., (2010), Maupas J.L. et al., (1983), Shan et al., (2013), Sudha M.R. et al., (2012), Szajewska H et al (2005), Zhang DM, et al (2017), Zhao et al (2014), Zojaji H., (2013).
Saccharomyces boulardii is widely used as a ‘yeast against yeast’ strategy to help inhibit the overgrowth of Candida yeasts in the body. The Candida genus of yeasts are natural residents of the human body; however, when conditions are appropriate for their proliferation, they can multiply and cause yeast infections known as Candidiasis (thrush).
Research indicates that S. boulardii secretes capric, caprylic, and caproic acids which inhibit the hyphae formation on the Candida yeast, preventing it from multiplying. It also reduces Candidial adhesion and biofilm formation, again reducing its ability to thrive and grow (Murzyn et al., 2010). A study showed that both S. boulardii, significantly inhibited Candida albicans adhesion to epithelial cell lines. The IL-8 gene expression by C. albicans-infected Caco-2 cells was suppressed by the addition of S. boulardii. These results indicate that S. boulardii affects C. albicans adhesion and reduces cytokine-mediated inflammatory host response. (Murzyn A. et al., 2010)
A prospective, randomised, comparative study was conducted on a total of 181 preterm infants with a gestational age of less than 32 weeks, and a birth weight of less than 3.3lbs. They were randomised into two groups, to receive either S. boulardii or the anti-fungal nystatin. Weekly skin and stool cultures were performed to determine colonisation, and weekly blood cultures taken to check for invasive infections. Two patients had Candida-positive blood cultures in the nystatin group, whereas no evidence of Candida was seen in the probiotic group. Additionally, feeding intolerance and clinical sepsis were significantly lower in the probiotic group than in the nystatin group (Demirel, et al., 2013).
NB: S. boulardii is not always recommended for those under the age of 1. Check with your probiotics supplier before giving to infants.
Further relevant studies: Algin C., et al. (2005), Berg et al., (1993), Berg R. et al., (1993), Ducluzeau, R., (1982), Jawhara, S., (2007), Kumar S. et al., (2013), Krasowska A. et al., (2009), Lherm T. et al., (2002), McFarland L. V., (2010), Tomičić, Z. et al., (2016).
The term antibiotic-associated diarrhoea (AAD) refers to a benign, self-limiting diarrhoea which often manifests following the use of antibiotics. Usually, no pathogens are identified and the diarrhoea is caused by changes in the balance and function of the gut flora. On the other hand, though it often occurs in connection with antibiotic use, Clostridium difficile infection causes diarrheal illnesses due to the toxins produced by this gram-positive anaerobic pathogenic bacteria.
A review of four studies conducted by Tung et al. (2009) assessed the effects of Saccharomyces boulardii on Clostridium difficile infection. Two of the studies considered cases of recurrent C. difficile issues in patients, and the other two studies looked at primary prevention of the infection in patients taking antibiotics. Although the first two studies yielded some interesting results and showed some positive trends occurring as a result of supplementation with S. boulardii, the second two studies failed to offer statistically relevant results. The review concluded, therefore, that S. boulardii may be effective for secondary prevention of C. difficile, and helps to prevent recurrence; however, more research is needed in order to ascertain its potential role in primary prevention of the infection. A similar conclusion was reached in a review by Surawicz (2003), who also suggested that there may be a role for S. boulardii as an adjunct in the treatment of recurrent Clostridium difficile- associated disease.
Further relevant studies: Buts J. et al., (1993), Castagliuolo I. et al., (1999), McFarland L.V., (2010), Pothoulakis, C. et al., (1993), Surawicz, C.M. et al., (1989), Surawicz, C.M. et al., (2000), Surawicz, C.M., (2003).
A significant role for the gut microflora in the pathogenesis of inflammatory bowel disease is now being identified, although the mechanisms involved are not fully understood (Guslandi M. et al., 2003). The possible therapeutic mechanisms of probiotics in intestinal inflammatory disorders include: antagonism against enteric pathogens; strengthening of the gut mucosal barrier; inhibition of the local secretion of inflammatory mediators; and stabilisation of local immunological activity (Kelesidis T 2012).
Guslandi et al. (2003) looked at the effect of S. boulardii on 25 patients with IBD, who were experiencing mild to moderate flare-ups of Ulcerative Ulcerative Colitis. To test the effects of the probiotic, the patients received 5 billion of S. boulardii in addition to their treatment of Mesalazine. A successful outcome was achieved in 17 of the patients. The authors concluded that the Saccharomyces boulardii did induce remission in the patients; however, due to the lack of a control group for comparison, more research is needed to further substantiate these results.
Further relevant studies: Bafutto et al (2013), Choi et al (2011), Garcia et al (2008), Garrido-Mesa J. et al., (2015), Guslandi M. (2000), Kabir et al (2011), McFarland L. V., (2010), Thomas S. et al., (2011), Pineton de Chambrun (2015).
Chronic infection with H. pylori can be a risk factor for ulcer disease, gastric adenocarcinoma not to mention painful. Therefore, eradication of H. pylori is a primary goal in patients that are symptomatic. The eradication rates achieved by classic triple therapy consisted from proton pump inhibitor and double antibiotic therapy are quite low and range from 60% to 80% This is due to resistance to antibiotics and to moderate patient compliance. Antibiotic-associated gastrointestinal side effects are a major cause for lower compliance.
Bin et al (2015) this trial looked at 194 children who were H. pylori positive. Saccharmoyces boulardii as given to the treatment group, and the control group was given a placebo. In the test group diarrhea occurred in 12 cases (11.76%), starting after approx. 6.25 days, lasting 3.17 days, and compliance with eradication treatment was 100%. In the control group, diarrhea occurred in 26 cases (28.26%), starting after 4.05 days, lasting 4.02 days, and in six cases eradication treatment was stopped prematurely. The researchers concluded therefore that Saccharomyces boulardii, although wouldn’t eradicate H. pylori on its own would help by reducing bacterial load and probably increase eradication rate due to increasing compliance to treatment. S. boulardii is, therefore, a useful adjuvant.
Further relevant studies: Homan & Orel (2015), Namkin et al (2016)
The anti-inflammatory potential of S. boulardii has attracted some attention in the field of probiotic research, and a particularly interesting study to demonstrate this activity was conducted with the co-operation of a group of HIV-positive patients. Imbalances in the gut microbiota are particularly concerning in such vulnerable groups of patients; gut dysbiosis has been linked to increased microbial translocation resulting in chronic inflammation in HIV patients.
A trial looked at the impact of probiotic supplementation on the composition of the gut microbiome (tested via faecal samples) in 44 HIV patients, who had been randomised to receive either S. boulardii or a placebo for three months. Compared to the placebo group, patients taking the probiotic had lower levels of some gut bacteria species, which were associated with systemic levels of bacterial translocation and inflammation markers. The authors concluded that Saccharomyces boulardii is able to benefit the gut microbe composition and systemic inflammation in HIV patients (Villar-Garcia J. et al, 2017).
Further relevant studies: Villar-Garcia J. et al (2015).
Research into the treatment of parasites with probiotics is still in its infancy, however, there is some evidence to suggest that Saccharomyces boulardii may be beneficial to those with a parasitic infection.
Further relevant studies: Dinleyici et al. (2009), Dinleyici (2011), Mansour-Ghanaei et al. (2003).
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Saccharomyces strain: Saccharomyces cerevisiae boulardii.
For more insights and professional updates on probiotics, please visit the Probiotic Professionals pages.
Abbas Z. et al., (2014), ‘Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: a randomized trial’, Eur J Gastroenterol Hepatol, 26(6):630-9.
Akil I. et al., (2006), ‘Influence of oral intake of Saccharomyces boulardii on Escherichia coli in enteric flora’, Pediatr Nephrol, 21(6):807-10.
Algin C., et al. (2005) ‘Effectiveness of Bombesin and Saccharomyces boulardii against the translocation of Candida albicans in the digestive tract in immunosuppressed rats’. Surgery Today, 35: 869.
Bafutto M. et al., (2013), ‘Treatment of diarrhea-predominant irritable bowel syndrome with mesalazine and/or Saccharomyces boulardii’,Arq Gastroenterol, 50(4):304-9.
Berg R. et al., (1993), ‘Inhibition of Candida albicans translocation from the gastrointestinal tract of mice by oral administration of Saccharomcyes boulardii’. J. Infect. Dis. 168(5):1314-8.
Billoo A.G. et al., (2006), ‘Role of a probiotic (Saccharomyces boulardii) in management and prevention of diarrhoea’, World J Gastroenterol, 12(28):4557-60.
Bin, Z., et al. (2015). The Efficacy of Saccharomyces boulardii CNCM I-745 in Addition to Standard Helicobacter pylori Eradication Treatment in Children. Pediatric Gastroenterology, Hepatology & Nutrition, 18(1), 17–22.
Blehaut, H. et al., (1989) ‘Disposition kinetics of Saccharomyces boulardii in man and rat’. Biopharm Drug Dispos, 10: 353–364.
Bruggencate T., (2015), ‘The effect of a multi-strain probiotic on the resistance toward Escherichia coli challenge in a randomized, placebo-controlled, double-blind intervention study’. European Journal of Clinical Nutrition, 69:385-391.
Burande M.A., (2013), ‘Comparison of efficacy of Saccharomyces boulardii strain in the treatment of acute diarrhea in children: A prospective, single-blind, randomized controlled clinical trial’, J Pharmacol Pharmacother, 4(3):205-8.
Buts J. et al., (1993), ‘Saccharomyces boulardii for Clostridium difficile-Associated Enteropathies in Infants’. Journal of Pediatric Gastroenterology and Nutrition, 16:419-425.
Buts J.P. et al., (1986), ‘Response of human and rat small intestinal mucosa to oral administration of S. boulardii’. Pediatr. Res., 20:192-6.
Can M. et al., (2006), ‘Prophylactic Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea: a prospective study’, Med Sci Monit, 12(4):19-22.
Canani R.B. et al., (2007), ‘Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations’, BMJ, 335(7615):340.
Castagliuolo, I. et al., (1999), ‘Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa’. Infect. Immun., 67(I): 302 - 307.
Cetina-Sauri, G. & Basto, S., (1994), ‘Therapeutic evaluation of Saccharomyces boulardii in children with acute diarrhea’. Annales de Pediatrie, 41(6):397-400.
Choi C.H. et al., (2011), ‘A randomized, double-blind, placebo-controlled multicenter trial of Saccharomyces boulardii in irritable bowel syndrome: effect on quality of life’, J Clin Gastroenterol, 45(8):679-83.
Cindoruk M., (2007), ‘Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study’. Helicobacter, 12(4):309-16.
Corrêa N.B. et al., (2011), ‘Treatment of acute diarrhea with Saccharomyces boulardii in infants’, J Pediatr Gastroenterol Nutr., 53(5):497-501.
Cremonini F. et al., (2002), ‘Effect of different probiotic preparations on anti-Helicobacter pylori therapy-related side effects: a parallel group, triple blind, placebo-controlled study’, Am J Gastroenterol, 97(11):2744-9.
Das S, et al. (2016) Efficacy and Safety of Saccharomyces boulardii in Acute Rotavirus Diarrhea: Double Blind Randomized Controlled Trial from a Developing Country. J Trop Pediatr. Dec;62(6):464-470
D'Souza, A. et al., (2002), ‘Probiotics in prevention of antibiotic associated diarrhoea: meta-analysis’. BMJ, 324:1361.
Demirel, G., et al., (2013), ‘Prophylactic Saccharomyces boulardii versus nystatin for the prevention of fungal colonization and invasive fungal infection in premature infants’. Eur. J. Pediatr., 172:1321.
Dias, R.S., et al (1995). Protective effect of Saccharomyces boulardii against the cholera toxin in rats. Braz. J. Med. Biol. Res. 28: 323- 325.
Dinleyici E. C. et al., (2009), ‘Clinical efficacy of Saccharomyces boulardii and met*******ole compared to met*******ole alone in children with acute bloody diarrhea caused by amebiasis: a prospective, randomized, open label study’, Am J Trop Med Hyg., 80(6):953-5.
Dinleyici E. C. et al., (2011), ‘Clinical efficacy of Saccharomyces boulardii or met*******ole in symptomatic children with Blastocystis hominis infection’, Parasitol Res., 108(3):541-5.
Dinleyici E.C. et al., (2015), ‘Saccharomyces boulardii CNCM I- 745 reduces the duration of diarrhoea, length of emergency care and hospital stay in children with acute diarrhoea’. Benef Microbes. 6(4):415-21.
Doron S. et al (2008) Probiotics for prevention of antibiotic-associated diarrhea 42 Suppl 2:S58-63 J Clin Gastroenterol.
Ducluzeau R. & Bensaada M., (1982), ‘Comparative effect of a single or continuous administration of Saccharomyces boulardii on the establishment of various strains of Candida in the digestive tract of gnotobiotic mice’. Annales de microbiologie. 133:491-501.
Duman D.G. et al., (2005), ‘Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacter pylori eradication’. Eur. J. Gastroenterol. Hepatol., 17(12):1357-61.
Erdeve, O. et al., (2004), 'The probiotic effect of S.boulardii in a pediatric age group'. Journal of Trop. Pediatr., 50(4):234-6.
Eren M. et al., (2010), ‘Clinical efficacy comparison of Saccharomyces boulardii and yogurt fluid in acute non-bloody diarrhoea in children: a randomized, controlled, open label study’, Am J Trop Med Hyg, 82(3):488-91.
Fidan I. et al., (2009), ‘Effects of Saccharomyces boulardii on cytokine secretion from intraepithelial lymphocytes infected by Escherichia coli and Candida albicans’. Mycoses, 52(1):29-34.
Gaón D. et al., (2003), ‘Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children’. Medicina, 63(4):293-8.
Garcia Vilela E. et al., (2008), ‘Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission’, Scand J Gastroenterol, 43(7):842-8.
Gotteland M. et al., (2005), ‘Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori.’Acta Paediatr., 94(12):1747-51.
Grandy G. et al., (2010), ‘Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children’. BMC Infectious Diseases, 10:253.
Guslandi M. et al., (2000), ‘Saccharomyces boulardii in Maintenance Treatment of Crohn's Disease’. Digestive Diseases & Sciences. 45(7):1462-1464.
Guslandi M. et al., (2003), ‘A pilot trial of Saccharomyces boulardii in ulcerative colitis’. European Journal of Gastroenterology & Hepatology. 15:697-698.
Garrido-Mesa J. et al., (2015), ‘A new therapeutic association to manage relapsing experimental colitis: Dox*****ine plus Saccharomyces boulardii’. Pharmalogical Research, 97:48-63.
Hafeez A. et al., (2002), ‘The efficacy of Saccharomyces boulardii in the treatment of acute watery diarrhea in children: a multicentre randomized controlled trial’, Journal of the College of Physicians and Surgeons Pakistan, 12(7):432-434.
Htwe K. et al., (2008), ‘Effect of Saccharomyces boulardii in the treatment of acute watery diarrhea in Myanmar children: a randomized controlled study’. Am. J. Trop. Med. Hyg., 78(2):214-6.
Hochter W. et al (1990) ‘Saccharomyces boulardii in acute adult diarrhea. Efficacy and tolerance of treatment’. Munchener Medizinische Wochenschrift; Vol. 132 (12) pp. 188-192.
Homan, M., & Orel, R. (2015). Are probiotics useful in Helicobacter pylori eradication? World Journal of Gastroenterology : WJG, 21(37), 10644–10653.
Hossain Md Nur et al., (2020) Identification and Growth Characterization of a Novel Strain of Saccharomyces boulardii Isolated From Soya Paste. Frontiers in Nutrition, 7:27.
Hurduc V. et al., (2009), ‘A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children’. Acta. Paediatr., 98(1):127-3.
Jawhara S. & Poulain D., (2007), ‘Saccharomyces boulardii decreases inflammation and intestinal colonisation by Candida albicans in a mouse model of chemically-induced colitis’. Medical Mycology, 45(8):691-700.
Kabir, M.A., et al., (2009), ‘Lack of Efficacy of Saccharomyces Boulardii in Diarrhea Predominant Irritable Bowel Syndrome–a Randomized Double–Blind, Placebo Controlled Clinical Trial’, European Journal of Scientific Research, 38(1): 104-109.
Kabir M. A. et al., (2011), ‘Role of Saccharomyces boulardii in diarrhea predominant irritable bowel syndrome’, Mymensingh Med J., 20(3):397-401.
Kelesidis T, Pothoulakis C.(2012) ‘Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders’ Therapeutic Advances in Gastroenterology. 2012;5(2):111-125.
Kirchhelle A. et al., (1996), ‘Treatment of persistent diarrhoea with Saccharomyces boulardii in returning travellers: results of a prospective study’. Fortschr. Med.114(11):136-140.
Klein, S.M. et al., (1993) ‘Recovery and elimination of the biotherapeutic agent, Saccharomyces boulardii, in healthy human volunteers’. Pharm Res, 10: 1615–1619.
Kurugol Z. & Koturoglu G., (2005), ‘Effects of Saccharomyces boulardii in children with acute diarrhea’. Acta Paediatrica, 94:44-47.
Kotowska M. et al., (2005), ‘Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea in children: a randomized double-blind placebo-controlled trial’. Alimentary Pharmacology & Therapeutics, 21(5):583–590.
Krasowska A. et al., (2009), ‘The antagonistic effect of Saccharomyces boulardii on Candida albicans filamentation, adhesion and biofilm formation’. FEMS Yeast Research, 9(8):1312-1321.
Kumar S. et al., (2013), ‘Probiotic use and prevalence of candidemia and candiduria in a PICU’. Pediatric Critical Care Medicine,14(9):e409-15.
Lherm T. et al., (2002), ‘Seven cases of fungemia with Saccharomyces boulardii in critically ill patients’. Intensive Care Med., 28(6):797-801.
Le Luyer B. et al., (2010), ‘A multicentric study of a lactose free formula supplemented with Saccharomyces boulardii in children with acute diarrhea’. Arch. Pediatr., 17(5):459-65.
McFarland L.V., (2010), ‘Systematic review and meta-analysis of Saccharomyces boulardii in adult patients’. World Journal of Gastroenterology, 16(18):2202–2222.
Mansour-Ghanaei F. et al., (2003), ‘Efficacy of Saccharomyces boulardii with antibiotics in acute amoebiasis’, World J Gastroenterol, 9(8):1832-3.
McFarland L.V. & Bernasconi P., (1993), ‘Saccharomyces boulardii: A Review of an Innovative Biotherapeutic Agent’. Microbial Ecology in Health and Disease, 6:157-171.
Matsubara VH., et al (2016) Probiotics as Antifungals in Mucosal Candidiasis. Clin Infect Dis. May Clin Infect Dis. 2016 May 1;62(9):1143-53
Maupas J.L. et al., (1983), ‘Treatment of Irritable Bowel Syndrome (IBS) Double Blind Trial of Saccharomyces boulardii’. Medecine Chirurgie Digestives, 12(1):77-79.
Murzyn et al., (2010), ‘Capric Acid Secreted by S. boulardii Inhibits C. Albicans’. Plos One, 5(8):
Murzyn A, et al. (2010) ‘The effect of Saccharomyces boulardii on Candida albicans-infected human intestinal cell lines Caco-2 and Intestin 407’. FEMS Microbiology Letters 310 (1) 17-23.
Namkin, K., et al. (2016). Saccharomyces Boulardii in Helicobacter Pylori Eradication in Children: A Randomized Trial From Iran. Iranian Journal of Pediatrics, 26(1), e3768.
Pineton de Chambrun G. et al., (2015), ‘A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome’. Dig. Liver Dis., 47(2):119-24.
Plein K, Hotz J. 1993 Therapeutic effects of Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn's disease with special respect to chronic diarrhea—a pilot study. Z Gastroenterol; 31: 129-134
Pothoulakis, C. et al., (1993), ‘Saccharomyces boulardii inhibits Clostridium difficile toxin: A binding and enterotoxicity in rat ileum’. Gastroenterology, 104(4):1108-1115.
Qamar et al., (2001), ‘Saccharomyces boulardii Stimulates Intestinal Immunoglobulin A Immune Response to Clostridium difficile Toxin A in Mice’. Infect. Immun., 69(4):2762–2765.
Shan L.S. et al., (2013), ‘Prevention and treatment of diarrhoea with Saccharomyces boulardii in children with acute lower respiratory tract infections’. Benef. Microbes, 4(4):329-34.
Sudha M.R. et al., (2012), ‘Oral consumption of potential probiotic Saccharomyces boulardii strain Unique 28 in patients with acute diarrhoea: a clinical report’. Benef. Microbes, 3(2):145-50.
Surawicz C.M. et al., (1989), ‘Treatment of Recurrent Clostridium difficile Colitis with Van****cin and Saccharomyces boulardii.’ The American Journal of Gastroenterology, 85(10).
Surawicz, C.M. et al., (2000), ‘The Search for a Better Treatment for Recurrent Clostridium difficile Disease: Use of High-Dose Van****cin Combined with Saccharomyces boulardii’. Clinical Infectious Diseases, 31:1012-1017.
Surawicz C.M., (2003), ‘Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans’. Best Practice & Research Clinical Gastroenterology, 17(5):775-783.
Szajewska H and Mrukowicz J.(2005) Meta-analysis: non-pathogenic yeast Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 22(5):365-72.
Thomas S. et al., (2011), ‘Anti-inflammatory effects of Saccharomyces boulardii mediated by myeloid dendritic cells from patients with Crohn's disease and ulcerative colitis’. American Journal of Physiology - Gastrointestinal and Liver Physiology, 301(6).
Tomičić Z. et al., (2016), ‘Probiotic yeast Saccharomyces boulardii (nom. nud.) modulates adhesive properties of Candida glabrata’. Med. Mycol., 54(8):835-845.
Tung J.M. et al., (2010), ‘Prevention of Clostridium difficile with Saccharomyces boulardii: A systematic review’. Canadian Journal of Gastroenterology, 23(12):817-821.
Villar-García J. et al., (2015), ‘Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial’, J Acquir Immune Defic Syndr., 68(3):256-63.
Villar-García J. et al., (2017), ‘Impact of probiotic Saccharomyces boulardii on the gut microbiome composition in HIV-treated patients: A double-blind, randomised, placebo controlled trial’. PLoS ONE, 12(4): e0173802.
Villarruel G. (2007), ‘Saccharomyces boulardii in acute childhood diarrhoea: a randomized, placebo-controlled study’. Acta Paediatr., 96(4):538-41.
Wan CM, et al (2017) A multicenter randomized controlled study of Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea in infants and young children. 4;55(5):349-354.
Wu (2007) Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol; 294, 1: G295-306.
Zengbin L et al., (2021) Which Probiotic Is the Most Effective for Treating Acute Diarrhea in Children? A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Nutrients, 13: 4319.
Zhao H. M. et al., (2014), ‘[Clinical effect of triple therapy combined with Saccharomyces boulardii in the treatment of Helicobacter pylori infection in children]’, Zhongguo Dang Dai Er Ke Za Zhi, 16(3):230-3.
Zhang DM, et al (2017) A prospective control study of Saccharomyces boulardii in prevention of antibiotic-associated diarrhea in the older inpatients. Jun 1;56(6):398-401.
Zojaji H. et al., (2013), ‘The efficacy and safety of adding the probiotic Saccharomyces boulardii to standard triple therapy for eradication of H. pylori: a randomized controlled trial’. Gastroenterol. Hepatol. Bed Bench, 6(1):S99-S104.
Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 10th January, 2024