As with many other strains in this database, this bacterial strain has been researched to assess its potential benefits for the relief of Irritable Bowel Syndrome (IBS) and the associated symptoms. The following studies suggest that it may do so because it has an anti-inflammatory effect in the body. It is important to note that this strain is also known by a few different names. This strain has actually recently undergone genome sequencing and whereas it was known beforehand as Bifidobacterium longum subsp. infantis 35624, the genome sequencing has actually caused it to be reclassified as Bifibobacterium longum subsp. longum 35634. Strictly speaking, therefore, this strain should come under the longum species however it is still much better known and commercially sold as an infantis strain, and it has been kept as such for the purpose of this Probiotics Database. This strain is also commercially known as Bifantis® (Altmann F, et al. 2016).
Bifidobacterium infantis 35624 is a food supplement with safety and survivability studies indicating it is able to reach the gut alive and is safe for human consumption. Lynseng-Williamson, K. (2017) produced an assessment of B. infantis 35624 as a food supplement, addressing tolerability across all available clinical trials, concluding ‘a tolerability and safety profile similar to that of placebo’.
A study by Charbonneau, D. et al (2013) found that after 8 weeks of oral supplementation with B. infantis 35624, the strain was recovered from stool samples, suggesting that the strain reaches the gut alive. Faecal levels of Bifidobacterium infantis 35624 were found to decline and return to baseline levels once oral supplementation ceased, indicating its transiency.
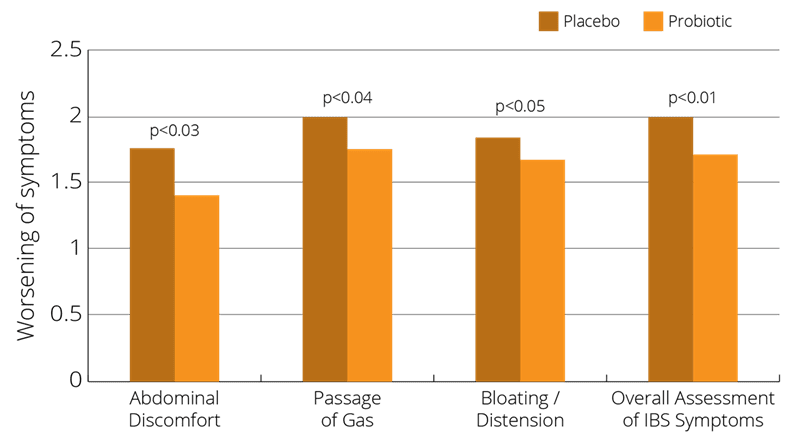
In a randomised, multiple-dosage, parallel, placebo-controlled, double-blind clinical trial, 75 male and female participants with various presentations of IBS (either constipation, diarrhoea or alternating symptoms) were given either a Lactobacillus salivarius strain or the Bifidobacterium infantis 35624 strain for a period of 8 weeks. Symptoms of IBS were assessed daily. Blood sampling for the measurement of cytokines IL-10 or IL-12 took place at the beginning and end of the study, and these were also compared against the cytokine levels of healthy volunteers. For the individual symptom scores, B. infantis 35624 was associated with a significant reduction in pain/discomfort, bloating/distention, and difficulty passing a bowel movement. Another important finding was that, also following B. infantis 35624 use, cytokine levels in the IBS subjects were similar to the levels in the healthy volunteers, while the levels were not significantly changed in either of the other treatment groups (O’Mahony et al., 2005).
Another large scale multicentre, randomised, multiple-dosage, parallel, placebo-controlled, double-blind clinical trial on women was conducted in order to determine the best dosage of Bifidobacterium infantis 35624 for IBS. The trial used 362 female subjects, all aged between18 to 65 years and diagnosed with IBS. The women were randomly split into groups and given one of three different doses of B. infantis 35624: 1 million, 100 million, or ten billion. B. infantis 35624 at the dosage of a hundred million had statistically lower symptom scores at week 4 of the treatment phase for abdominal pain/discomfort, bloating/distention, sense of incomplete evacuation, passage of gas, straining, and bowel habit satisfaction than those receiving placebo (Whorwell et al., 2006).
Further relevant studies: Bairead et al (2005), Brenner (2009a), Brenner et al (2009b), Chang et al (2005), Charbonneau et al., (2005a), Charbonneau et al., (2005b), Charbonneau et al., (2005c), Charbonneau et al., (2005d), Chen K-S et al (2005), Mayer (2008), O’Mahony et al., (2005), Quigley et al (2005a), Quigley et al (2005b).
In a study authored by Konieczna (2012), cytokine secretion and T cell expression were monitored in individuals who were given Bifidobacterium infantis 35624. It was found that B. infantis administration in humans promotes immuno-regulatory responses, suggesting that this microbe may have therapeutic utility in patients with inflammatory disease. These findings link nutrition, gut microbiota and the induction of tolerance within the gastrointestinal mucosa.
Bifidobacterium infantis 35624 was also found to modulate inflammatory processes beyond the gut. Groeger et al (2013) assessed the impact of oral administration of B. infantis 35624, for 6‒8 weeks on inflammatory biomarker and plasma cytokine levels in patients with ulcerative colitis, chronic fatigue syndrome and psoriasis in three separate randomised, double-blind, placebo-controlled interventions. In addition to this, the effect of B. infantis 35624 on immunological biomarkers in healthy subjects was assessed. This strain of bacteria resulted in a reduced plasma CRP levels in all three inflammatory disorders. Compared with the placebo results, additionally plasma TNF-α was reduced in chronic fatigue syndrome and psoriasis, while IL-6 was reduced in ulcerative colitis and chronic fatigue syndrome. These results show the ability of this microbe to reduce pro-inflammatory markers both in the gut and systemically.
Further relevant studies: Gad et al., (2011), Johnson et al., (2011), Konieczna et al., (2013), Scully et al (2013) Symonds et al., (2012), van der Kleij et al., (2008), O’Mahony et al., (2005), Sheil et al (2007), Shanahan et al (2006), Sommerfield et al (2003), Symonds et al (2007), Wall et al (2010), von Wright et al (2002).
This strain has been used in rat studies conducted by Desbonnet et al., (2008) (2010), to assess the effects of probiotics on behaviour and mood. Results suggested that probiotic treatment resulted in normalisation of the immune response, reversal of behavioural issues and reduction in depressive symptoms.
In the same O’Mahony et al (2005) trial that is described in the IBS section above, it was found that the ratio of anti-inflammatory to pro-inflammatory cytokines in the gut was normalised. This suggests an immune-modulating role for this microorganism, in this disorder.
Further relevant studies: Davies et al (2009), McCarthy et al (2003), O’Callaghan et al (2002), O’Callaghan et al (2003), O’Callaghan et al (2004), O’Mahony et al (2008), O’Mahony et al (2005), O’Mahony et al (2006), Sheil et al.(2006a), Sheil et al., (2006b) Shilling et al., (2005), Sibartie et al., (2009).
A pilot study by Frech et al (2011) indicated a role for probiotics to support systemic sclerosis (SSc) patients with systemic sclerosis-associated re-flux and distention/ bloating symptoms. Improvements in symptoms and total gastrointestinal tract (GIT) disease scales were noted.
Some protection against Salmonella infection and its damaging effects were noted in a variety of murine studies - O’Mahony et al., (2002), O’Mahony et al., (2004), O'Hara et al, (2006), Sommerfield et al., (2005).
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 25th May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Bifidobacterium lactis strains: Bifidobacterium lactis Bi-07®, Bifidobacterium lactis BB-12®, Bifidobacterium lactis HN019 and Bifidobacterium lactis Bl-04®.
Bifidobacterium infantis strains: Bifidobacterium infantis 35624.
Bifidobacterium breve strains: Bifidobacterium breve M-16V®.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Altmann F, et al. (2016), 'Genome Analysis of Characterisation of the Exopolysaccharide Produced by Bifidobacterium longum subsp.longum 35624TM. Seleem MN, ed. PLoS ONE. 11(9):E0162983
Bairead E. el al.(2005), 'Alterations in immunological gene expression from colonic biopsies in female IBS patients following probiotic consumption'. Gastroenterology, 128(44)
Brenner D.M., & Chey W.D. (2009a), ‘Bifidobacterium infantis 35624: A novel probiotic for the treatment of IBS’. Rev Gastroenterol Disord. 9 (1):7-15.
Brenner D.M., et al (2009b). ‘The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review’. Am J Gastroenterol. 104 (4):1033-49.
Chang K., et al (2005), ‘Modulation of cytokine profiles in healthy and IBS subjects following supplementation with the unique probiotic strain, Bifidobacterium infantis 35624’, P&G Health Sciences Institute Publication 2005, Gastroenterology,128, (4 Suppl 2) pp.A661
Charbonneau D., et al., (2005a) ‘Impact of Bifidobacterium infantis 35624 on fecal flora from healthy and IBS subjects in a chemostat model’. Gastroenterology, 128: A-661.
Charbonneau D.L., et al. (2005b) ‘Fecal flora effects following oral supplementation with Bifidobacteria infantis 35624 in healthy and IBS subjects’, World Congress of Gastroenterology; September 2005; Montreal, Canada.
Charbonneau D. et al (2005c) Impact of Bifidobacterium infantis 35624 on fecal flora from healthy and IBS subjects in a chemostat model. Gastroenterology, 128(4 Suppl 2):A660-61.
Charbonneau D.L. et al (2005d), ‘Fecal flora effects following oral supplementation with Bifidobacteria infantis 35624 in healthy and IBS subjects’, Can J Gastroenterol. 19 (Suppl SB) pp.R1097.
Charbonneau D., et al (2006) ‘Development of Strain-Specific Molecular Method for the Identification of Bifidobacteria infantis 35624’, Gastroenterology 130(4) S2 pp.314
Charbonneau D, et al (2013) Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes. 2013; 4(3):201-211.
Chen K-S, et al (2005), ‘Oral administration with a unique probiotic strain, Bifidobacterium infantis 35624, to IBS subjects normalizes their systemic immunological imbalance in cytokine profile toward healthy subjects’, Can J Gastroenterol. 19 (Suppl SB):R.1112.
Ciprandi G, et al. (2022) The Probiotics in Pediatric Asthma Management (PROPAM) study. Ann Allergy Asthma Immunol. 129(1):111-113.
Desbonnet L. et al (2008), ‘The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat’, Journal of Psychiatric Research, 43(2):164-74.
Davies J.M, et al (2009), ‘Bacterial signalling overrides cytokine signalling and modifies dendritic cell differentiation’, Immunology, 128 (1 Suppl)
Desbonnet L. et al, (2010), ‘Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression’, Neuroscience, 170(4) pp.1179-88.
Frech T.M. et al (2011). Probiotics for the treatment of systemic sclerosis-associated gastrointestinal bloating/distension. Clin Exp Rheumatol, 29(2 Suppl 65):S22-5.
Gad M. et al (2011), ‘Regulation of the IL-10/IL-12 axis in human dendritic cells with probiotic bacteria’. FEMS Immunol Med Microbiol., 63(1) :93-107.
Groeger, D. (2013), ‘Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut’, Gut Microbes. 4 (4): 325-339.
Hoy-Schulz YE et al (2016) Safety and acceptability of Lactobacillus reuteri DSM 17938 and Bifidobacterium longum subspecies infantis 35624 in Bangladeshi infants: a phase I randomized clinical trial. BMC Complement Altern Med. 16:44.
Johnson A.C. et al (2011), ‘Effects of Bifidobacterium infantis 35624 on Post-Inflammatory Visceral Hypersensitivity in the Rat’. Dig Dis Sci. 56(11):3179-86.
Konieczna P. et al (2013), ‘Portrait of an immuno-regulatory Bifidobacterium’. Gut Microbes 3(3): 261-266.
Konieczna P. et al. (2012) ‘Bifidobacterium infantis 35624 administration induces Foxp3 T Regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells’, Gut. 61(3):354-66.
Lynseng-Williamson, K. (2017) Bifidobacterium infantis 35624 as a probiotic dietary supplement: a profile of its use. Drugs & therapy perspectives 33 (3)
Mayer E.A. (2008), ‘Clinical practice. Irritable bowel syndrome’, N Engl J Med. Apr 17, 358 (16):1692-9.
McCarthy J. et al (2003), ‘Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance’, Gut., 52(7) :975-80.
O’Callaghan L. et al (2002), ‘Human cytokine production by mesenteric lymph node cells in response to probiotic and pathogenic bacteria’, Gastroenterology, 122: A151
O’Callaghan L. et al (2003), ‘Differential cytokine response of cells derived from different lymphoid compartments to commensal and pathogenic bacteria’, Gastroenterology, 124: A339
O’Callaghan L. et al (2004), ‘Cytokine responses in different lymphoid compartments in IL-10/ko mice fed Bifidobacterium infantis’, Gastroenterology. 2004:126:
O'Hara AM, et al, (2006), 'Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius', Immunology, Jun, 118(2): 202-15.
O’Mahony L, et al (2002), ‘Multi-organism comparison of probiotic performance in a murine model of Salmonella typhimurimum infection’, Gastroenterology, 122:T1337
O’Mahony L, et al (2004), ‘Mechanism of probiotic/commensal mediated protection against invasive Salmonella infection’, Gastroenterology. 2004:126
O’Mahony L. et al (2005), ‘Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles, Gastroenterology, 128(3):541-51.
O’Mahony L. et al (2005), ‘Bifidobacteria induced cytokine production in vitro as predictive biomarkers of in vivo performance in the lymphocyte transfer model of IBD’, Gastroenterology, 128 (4 Suppl 2):A619
O’Mahony L. et al (2006), ‘Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans’, Am J Physiol Gastrointest Liver Physiol. 290 (4):G839-45.
O’Mahony C. (2008), ‘Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation’, PLoS Pathog. 4(8):e1000112.
Schiavi E, (2016) The surface associated exopolysaccharide of Bifidobacterium longum 35624 plays an essential role in dampening host pro-inflammatory responses and in repressing local TH17 responses.Appl Environ Microbiol. Oct 7.
Scully P. et al (2013), ‘Bifidobacterium infantis suppression of Peyer’s patch MIP-1α and MIP-1β secretion during Salmonella infection correlates with increased local CD4+CD25+ T cell numbers’, Cell Immunol.,281(2):134-140.
Shanahan F. et al and Progid Investigators, (2006), ‘A one year, randomised, double-blind, placebo controlled trial of a Lactobacillus or a Bidifobacterium probiotic for maintenance of steroid-induced remission of ulcerative colitis’, Gastroenterology, 130 (4 Suppl 2):A44.
Sheil B. et al (2007), ‘Probiotic effects on inflammatory bowel disease’, J Nutrition, 137(3 Suppl 2):819S-24S.
Sheil B. et al (2006), ‘Consumption of the probiotic Bifidobacterium infantis 35624 influences subsequent immune responses to in vitro challenge of isolated murine Peyer’s patch cells with bacterial stimuli’,Gastroenterology, 130(4 Suppl 2):A371.
Sheil B. et al (2006), ‘Role of interleukin (IL-10) in probiotic-mediated immune modulation: an assessment in wild-type and IL-10 knock-out mice’, Clin Exp Immunol., 144(2):273-80.
Shilling D. et al (2005), ‘Bacterial stimulation combined with heat shock protein targeting induces potent dendritic cell anti-tumor responses in a murine model. Gastroenterology. 128:A178 (Abstract S1250)
Sibartie S. et al (2009), ‘Modulation of pathogen-induced CCL20 secretion from HT-29 human intestinal epithelial cells by commensal bacteria’, BMC Immunol., 10:54.
Sommerfield D. et al (2003), ‘Influence of Bifidobacterium infantis feeding on secretagogue response and gut barrier function in rats recovering from colitis’, Gastroenterology, 124:A311
Sommerfield D. et al (2005), ‘Effect of probiotic feeding on Salmonella translocation in a Mouse model’, Gastroenterology, 128:A120. (Abstract 770)
Symonds E.L. et al (2012), ‘Bifidobacterium infantis 35624 protects against Salmonella-induced reductions in digestive enzyme activity in mice by attenuation of the host inflammatory response’. Clin Transl Gastroenterol., 3:e15.
Symonds E.L. et al (2007), ’Probiotic prevention of digestive enzyme damage in a mouse model of Salmonella’, Gastroenterology, 132(4 Suppl 2):A714-5.
Quigley E.M.M., et al and the PROGID investigators (2006), ‘Safety and tolerability of the probiotic organism Bifidobacterium infantis 35624: clinical experience and molecular basis’, Gastroenterology, 130(4) S2:493
Quigley E.M. et al (2005), 'Probiotic use results in normalization of bowel movement frequency in IBS: results from a clinical trial with the novel probiotic Bifidobacteria infantis 35624’, Am J of Gastroenterology, 100(9 Suppl S):S326
Quigley E.M. et al (2005), ‘Who is the responder to probiotic therapy in IBS? Data from a controlled clinical trial with Bifidobacterium infantis 35624’, Am J Gastroenterol., 100(9) Suppl:A325-326
Van der Kleij H. et al (2008), ‘Protective effects of Lactobacillus reuteri and Bifidobacterium infantis in murine models for colitis do not involve the vagus nerve’, Am J Physiol Regul Integr Comp Physiol., 295(4):R1131-7.
Von Wright A. et al (2002) ‘The survival and colonic adhesion of Bifidobacterium infantis in patients with ulcerative colitis’, International Dairy Journal, 12:197-200.
Wall R. et al (2010), ‘Impact of administered Bifidobacterium on murine host fatty acid composition’, Lipids, 45(5):429-36.
Whorwell P. et al (2006), ‘Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome’, American Journal of Gastroenterology, 101: 1581-1590.